The first superconducting Type 2 compound, an alloy of lead and bismuth, was fabricated in 1930 by W. de Haas and J. Voogd. But, was not recognized as such until later, after the Meissner effect had been discovered. This new category of superconductors was identified by L.V. Shubnikov at the Kharkov Institute of Science and Technology in the Ukraine in 1936(1) when he found two distinct critical magnetic fields (known as Hc1 and Hc2) in PbTl2. The first of the oxide superconductors was created in 1973 by DuPont researcher Art Sleight when Ba(Pb,Bi)O3 was found to have a Tc of 13K. The superconducting oxocuprates followed in 1986.
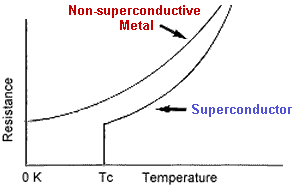
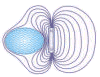
Type 2 superconductors - also known as the "hard" superconductors - differ from Type 1 in that their transition from a normal to a superconducting state is gradual across a region of "mixed state" behavior. Since a Type 2 will allow some penetration by an external magnetic field into its surface, this creates some rather novel mesoscopic phenomena like superconducting "stripes" and "flux-lattice vortices". While there are far too many to list in totality, some of the more interesting Type 2 superconductors are listed below by similarity and with descending Tc's. Where available, the lattice structure of the system is also noted.
| (Tl4Ba)Ba2MgCu8O13+ (As a 9223 structure) (Tl4Ba)Ba2Mg2Cu7O13+ (As a 9223 structure) (Tl4Ba)Ba2Ca2Cu7O13+ (As a 9223 structure) (Tl4Ba)Ba4Ca2Cu10Oy (As a 9212/2212C intergrowth.) Tl5Ba4Ca2Cu10Oy (As a 9212/2212C intergrowth.) (Sn5In)Ba4Ca2Cu11Oy (As a B212/2212C intergrowth.) (Sn5In)Ba4Ca2Cu10Oy (As a B212/1212C intergrowth.) Sn6Ba4Ca2Cu10Oy (As a B212/1212C intergrowth.) (Sn1.0Pb0.5In0.5)Ba4Tm6Cu8O22+ (As a 1256/1212 intergrowth.) (Sn1.0Pb0.5In0.5)Ba4Tm5Cu7O20+ (As a 1245/1212 intergrowth.) (Sn1.0Pb0.5In0.5)Ba4Tm4Cu6O18+ (As a 1234/1212 intergrowth) Sn3Ba4Ca2Cu7Oy (As a 5212/1212C intergrowth.) | ~265 K ~258 K ~254 K ~242 K ~233 K ~218 K ~212 K ~200 K ~195 K ~185 K ~163 K ~160 K |  |
(Hg0.8Tl0.2)Ba2Ca2Cu3O8.33 HgBa2Ca2Cu3O8 HgBa2Ca3Cu4O10+ HgBa2(Ca1-xSrx)Cu2O6+ HgBa2CuO4+ | 138 K* 133-135 K 125-126 K 123-125 K 94-98 K |
Lattice: TET
* Note: As a result of a topological "defect", Hg will also go into the Cu atomic sites. Thus, the volume fraction of the intended structure type is considerably less than 100%.
Tl2Ba2Ca2Cu3O10 (Tl1.6Hg0.4)Ba2Ca2Cu3O10+ TlBa2Ca2Cu3O9+ (TlSn)Ba4TmCaCu4Ox (Tl0.5Pb0.5)Sr2Ca2Cu3O9 Tl2Ba2CaCu2O6 TlBa2Ca3Cu4O11 TlBa2CaCu2O7+ Tl2Ba2CuO6 TlSnBa4Y2Cu4Ox | 127-128 K 126 K 123 K ~121 K (Superconductors.ORG - 2005) 118-120 K 118 K 112 K 103 K 95 K 86 K (Superconductors.ORG - 2007) |
Lattice: TET
| Sn4Ba4(Tm2Ca)Cu7Ox Sn2Ba2(Tm0.5Ca0.5)Cu3O8+ SnInBa4Tm3Cu5Ox Sn3Ba4Tm3Cu6Ox Sn3Ba8Ca4Cu11Ox SnBa4Y2Cu5Ox Sn4Ba4Tm2YCu7Ox Sn4Ba4TmCaCu4Ox Sn4Ba4Tm3Cu7Ox Sn2Ba2(Y0.5Tm0.5)Cu3O8+ Sn3Ba4Y2Cu5Ox SnInBa4Tm4Cu6Ox Sn2Ba2(Sr0.5Y0.5)Cu3O8 Sn4Ba4Y3Cu7Ox | ~127 K (TmTm-Ca structure only) ~115 K (Superconductors.ORG - 2005) ~113 K (Superconductors.ORG - 2005) 109 K (Superconductors.ORG - 2007) 109 K (One-of-a-Kind Resonant - 2006) 107 K (Superconductors.ORG - 2007) ~104 K (First Hi-Tc Reentrant - 2007) ~100 K (Superconductors.ORG - 2007) ~98 K (Superconductors.ORG - 2006) ~96 K (Superconductors.ORG - 2007) ~91 K (Superconductors.ORG - 2006) 87 K (Superconductors.ORG - 2005) 86 K (Aleksandrov, et al - 1989) ~80 K (Superconductors.ORG - 2005) |
| Bi1.6Pb0.6Sr2Ca2Sb0.1Cu3Oy Bi2Sr2Ca2Cu3O10*** Bi2Sr2CaCu2O9*** Bi2Sr2(Ca0.8Y0.2)Cu2O8 Bi2Sr2CaCu2O8 | 115 K (thick film on MgO substrate) 110 K 110 K 95-96K 91-92K |
Lattice: ORTH*** Though not always listed as a component, a small amount of Lead (x=.2-.26) is often used with Bismuth compounds to help facilitate a higher-Tc crystalline phase.
| (Ca1-xSrx)CuO2 YSrCa2Cu4O8+ (Ba,Sr)CuO2 BaSr2CaCu4O8+ (La,Sr)CuO2 | 110 K 101 K (Superconductors.ORG - 2007) 90 K 90 K (Superconductors.ORG - 2007) 42 K |
| Pb3Sr4Ca3Cu6Ox Pb3Sr4Ca2Cu5O15+ (Pb1.5Sn1.5)Sr4Ca2Cu5O15+ Pb2Sr2(Ca, Y)Cu3O8 | 106 K (Superconductors.ORG - 2007) 101 K (Superconductors.ORG - 2005) ~95 K (Superconductors.ORG - 2006) 70 K (Cava, et al - 1989) |
| AuBa2Ca3Cu4O11 AuBa2(Y, Ca)Cu2O7 AuBa2Ca2Cu3O9 | 99 K (Kopnin, et al - 2001) 82 K 30 K |
Lattice: ORTH
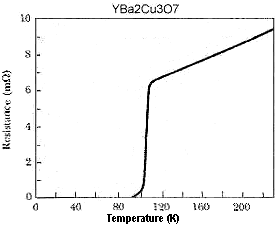
YBa3Cu4Ox (9223C structure) YCaBa3Cu5O11+ (Y0.5Lu0.5)Ba2Cu3O7 (Y0.5Tm0.5)Ba2Cu3O7 Y3Ba5Cu8Ox Y3CaBa4Cu8O18+ (Y0.5Gd0.5)Ba2Cu3O7 Y2CaBa4Cu7O16 Y3Ba4Cu7O16 Y2Ba5Cu7Ox NdBa2Cu3O7 Y2Ba4Cu7O15 GdBa2Cu3O7 YBa2Cu3O7 TmBa2Cu3O7 YbBa2Cu3O7 YSr2Cu3O7 Lattice: TET | 177 K (Superconductors.ORG - 2009) 107 K (Superconductors.ORG - 2010) 107 K (Superconductors.ORG - 2005) 105 K (Superconductors.ORG - 2005) 105 K (Superconductors.ORG - 2008) 99 K (Superconductors.ORG - 2010) 97 K (Superconductors.ORG - 2005) 96 K (Superconductors.ORG - 2006) 96 K (Superconductors.ORG - 2005) 96 K (Superconductors.ORG - 2008) 96 K 95 K 94 K 92 K (See above graphic) 90 K 89 K 62 K |
Comment: "1-2-3" superconductors actually have the 1212C structure. Thus, the formula for YBCO could be written CuBa2YCu2O7.
GaSr2(Ca0.5Tm0.5)Cu2O7 Ga2Sr4Y2CaCu5Ox Ga2Sr4Tm2CaCu5Ox La2Ba2CaCu5O9+ (Sr,Ca)5Cu4O10 GaSr2(Ca, Y)Cu2O7 (In0.3Pb0.7)Sr2(Ca0.8Y0.2)Cu2Ox (La,Sr,Ca)3Cu2O6 La2CaCu2O6+ (Eu,Ce)2(Ba,Eu)2Cu3O10+ (La1.85Sr0.15)CuO4 SrNdCuO**** (La,Ba)2CuO4 (Nd,Sr,Ce)2CuO4 Pb2(Sr,La)2Cu2O6 (La1.85Ba.15)CuO4 | 99 K (Superconductors.ORG - 2006) 85 K (Superconductors.ORG - 2006) 81 K (Superconductors.ORG - 2006) 79 K (Saurashtra Univ., Rajkot, India - 2002) 70 K 70 K 60 K 58 K 45 K 43 K 40 K 40 K 35-38 K 35 K 32 K 30 K (First HTS ceramic SC discovered - 1986) |
Comment: All of the above are copper perovskites, even though their metal-to-oxygen ratios are not exactly 2-to-3. The best performers are those compounds that contain one or more of the electron-emitters BaO, SrO or CaO, along with a Period 6 heavy metal like Mercury, Thallium, Lead, Bismuth, or Gold.
GdFeAsO1-x (Ca,Sr,Ba)Fe2As2 LiFeAs | 53.5 K (Highest Tc iron-based compound) 38 K 18 K |
MgB2 Ba0.6K0.4BiO3 | 39 K (Highest Tc Non-Fullerene Alloy) 30 K (First 4th order phase compound) |
| Nb3Ge Nb3Si Nb3Sn Nb3Al V3Si Ta3Pb V3Ga Nb3Ga V3In | 23.2 K 19 K 18.1 K 18 K 17.1 K 17 K 16.8 K 14.5 K 13.9 K |
Lattice: A15 Comment: Among the binary alloys, these are some of the best performers; combining Group 5B metals in a ratio of 3-to-1 with 4A or 3A elements.
| PuCoGa5 | 18.5 K (First SC transuranic compound) |
| NbN | 16.1 K |
Comment: After NbTi (below) NbN is the most widely used low-temperature superconductor.
| Nb0.6Ti0.4 MgCNi3 | 9.8 K (First superconductive wire) 7-8 K (First all-metal perovskite superconductor) |
| C Nb Tc V | 15 K (as highly-aligned, single-walled nanotubes) 9.25 K 7.80 K 5.40 K |
Lattice: C=Fullerene, Nb=BCC, Tc=HEX, V=BCC
Comment: These four are the only elemental Type 2 superconductors.
| RuSr2(Gd,Eu,Sm)Cu2O8 ErNi2B2C YbPd2Sn UGe2 URhGe2 AuIn3 | Tc ~58 K (Ruthenium-oxocuprate) Tc 10.5 K (Nickel-Borocarbide) Tc ~2.5 K (Heusler compound) Tc ~1K (Heavy fermion) Tc ~1K ( " ) Tc 50 uK |
Comment: The above 6 compounds are all rare ferromagnetic superconductors.
| Sr.08WO3 Tl.30WO3 Rb.27-.29WO3 | 2-4 K (Tungsten-bronze) 2.0-2.14 K (") 1.98 K (") |
Lattice: TET
| SrTiO3 | 0.35 K |
Comment: This is the first oxide insulator found to be superconductive